

— Blogs —
—Products—
 Consumer hotline +8618073152920
Consumer hotline +8618073152920 WhatsApp:+8615367865107
Address:Room 102, District D, Houhu Industrial Park, Yuelu District, Changsha City, Hunan Province, China
Product knowledge
Time:2024-07-27 18:08:04 Popularity:5297
Soil pH is an important indicator of soil chemistry, reflecting the relative concentrations of hydrogen ions (H+) and hydroxide ions (OH) in the soil, and is usually based on soil moistened with a 0.01 mol/L solution of potassium chloride at 25°C. pH ranges from 0 (strongly acidic) to 14 (strongly alkaline), with a neutral pH value of about 7. Soil pH is an important measure of the degree of acidity or alkalinity of the soil. Soil pH is an important indicator of the acidity or alkalinity of the soil and has an extremely important impact on agricultural production:
1. Affects plant growth: Different plants have different adaptations to soil pH. Different plants have different adaptations to soil pH. Unsuitable soil pH may result in slow growth, reduced yields, or even death of plants. Some plants such as Rhododendrons and Blueberries prefer acidic soils, while others such as peas and apple trees prefer alkaline soils. The right pH helps plants to absorb essential nutrients.
2. Nutrient availability: Soil pH affects the form and availability of nutrients (e.g., nitrogen, phosphorus, potassium, calcium, magnesium, etc.) in the soil. For example, soil that is too acidic or too alkaline will affect the solubility of trace elements, which in turn will affect plant uptake.
3. Microbial activity: Soil pH affects the type and activity of soil microorganisms. A suitable pH helps to maintain the survival of beneficial microorganisms and promotes the decomposition of organic matter and nutrient cycling.
4. Soil structure and texture: Soil pH can affect the charge of soil colloids, which in turn affects soil structure and the stability of aggregates. At the right pH, the soil forms a better structure that favours water and air infiltration and root development.
5. Pesticide and fertiliser efficiency: Soil pH affects the effectiveness of pesticides and fertilisers. An unsuitable pH may lead to fertiliser failure or pesticide decomposition, reducing their effectiveness.
6. Behaviour of soil contaminants: Soil pH can affect the activity, mobility and bioavailability of heavy metals and other contaminants. For example, in acidic soils, some heavy metals may be more soluble and taken up by plants, while in alkaline soils they may be present in insoluble forms.
7. Agricultural management decisions: Knowledge of soil pH can help farmers to make informed management decisions, such as choosing the right crop varieties, the type and amount of fertiliser to be applied, and making the necessary soil conditioning, such as applying lime to raise the soil pH or sulphur to lower it.
Therefore, soil pH not only affects the chemical and biological properties of the soil, but is also directly related to the efficiency and sustainability of agricultural production. By regularly testing and adjusting soil pH, soil conditions can be optimised to promote healthy crops.
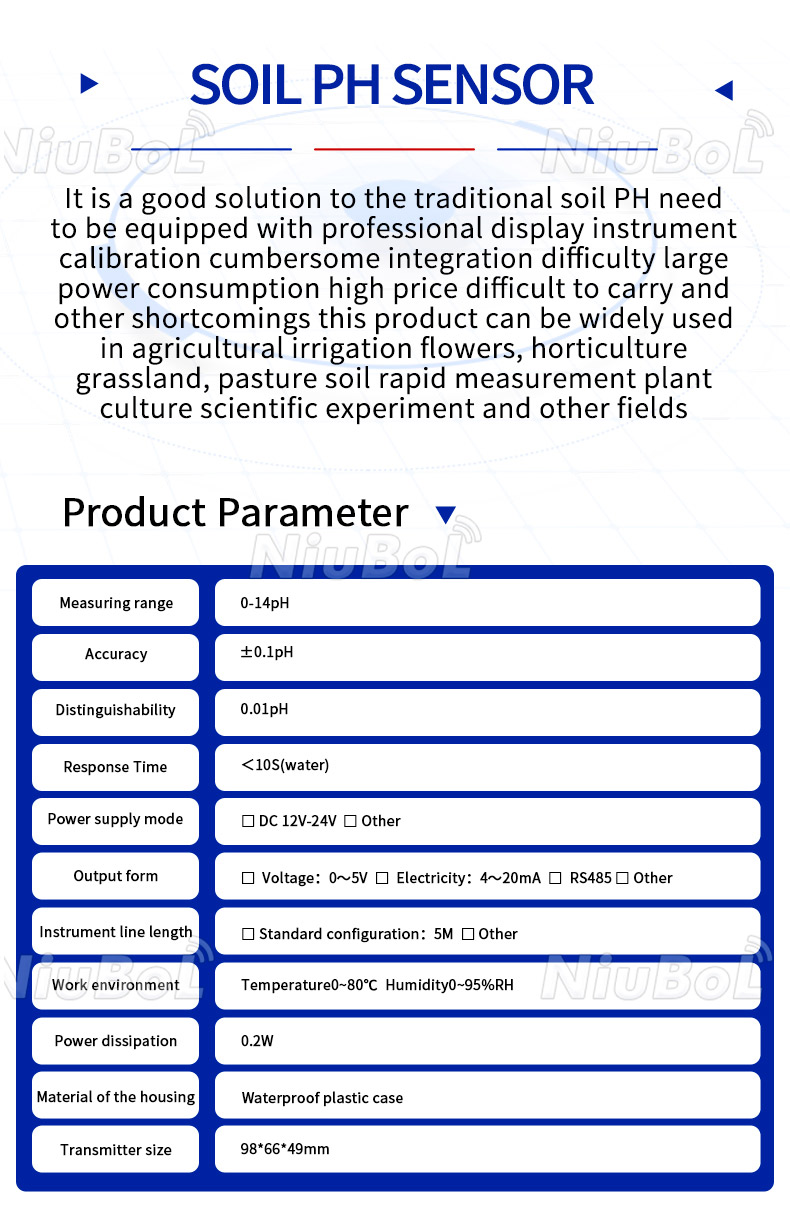
The Soil pH Sensor is a sensor specifically designed to measure soil acidity and alkalinity. It is capable of monitoring the pH of the soil in real time and usually consists of one or more electrodes that are inserted into the soil to determine the soil's acidity or alkalinity by measuring the concentration of hydrogen ions in the soil.
Soil pH sensor main features:
1. Measuring range: the measuring range of soil pH sensor is usually between 0-14, which can cover all possible pH values of soil.
2. Accuracy: Highly accurate soil pH sensors can provide a measurement accuracy of ±0.1 pH units.
3. Stability: High quality soil pH sensors have good stability and are less affected by environmental factors such as temperature and humidity.
4. Response time: soil pH sensors can respond quickly to changes in soil pH.
5. Durability: soil pH sensors used in agricultural sites usually need to have good durability to adapt to different soil and environmental conditions.

Soil pH Sensor Working Principle:
Soil pH sensors work on the principle of potential difference based on ion-selective electrodes. When the electrodes are inserted into the soil, the H+ and OH- ions in the soil react with the electrodes to produce a potential signal corresponding to the pH value. This signal is amplified and processed to be displayed as the pH value of the soil.
There are two main types of soil pH sensors:
1. Glass Electrode: This is the most common type of soil pH sensor and consists of a glass membrane with an indicator electrode consisting of an internal electrolyte, and a reference electrode. This type of sensor usually requires periodic calibration to ensure the accuracy of the measurements.
2. Electrochemical enzyme sensors: These sensors use enzymes to detect chemicals in the soil and determine the pH of the soil by measuring the activity of the enzymes. This type of sensor usually has higher stability and lower drift, but may require special calibration and maintenance.
How to use a soil pH sensor:
1. calibration: before using a soil pH sensor, calibration is usually required to ensure accurate measurements.
2. Insertion into the soil: Insert the electrode portion of the sensor into the soil, usually to a certain depth to obtain an accurate reading.
3. reading data: read the soil pH value through a connected display device or data acquisition system.
4. Data recording: Record the measurement data for subsequent analysis and management.
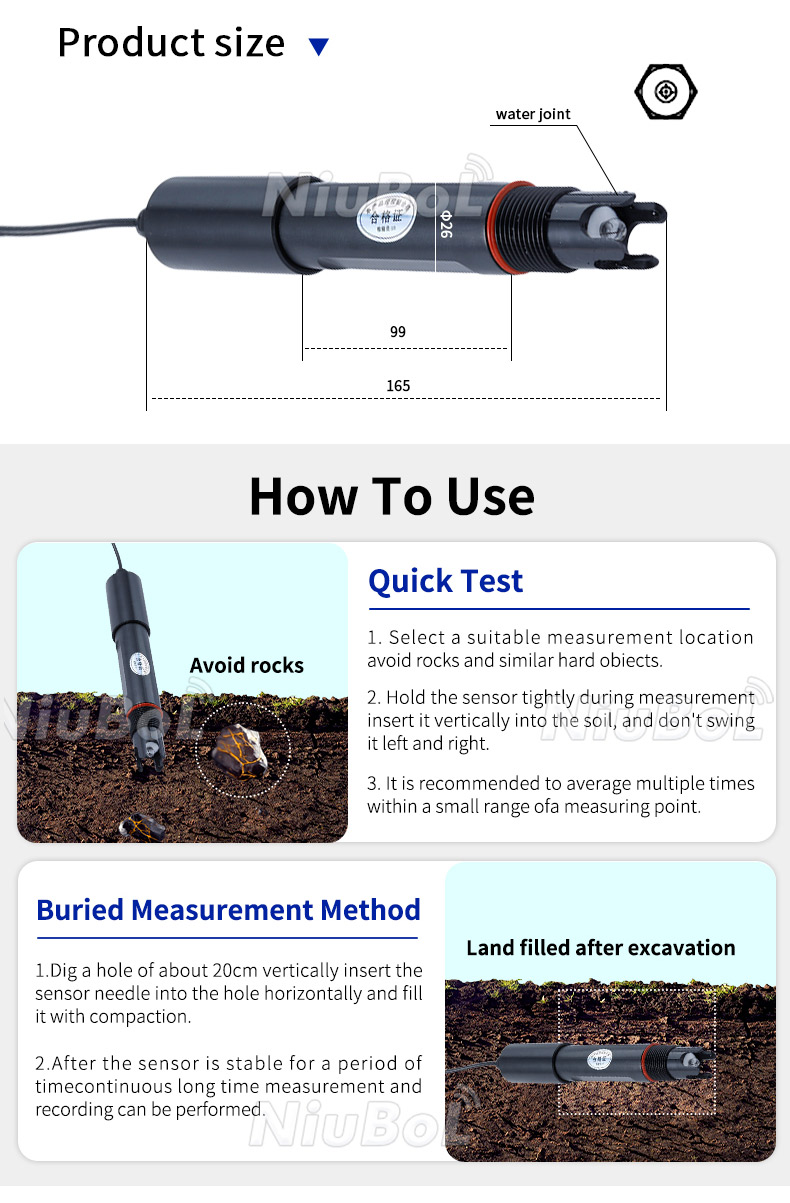
Soil pH sensor is widely used in agriculture, environmental monitoring and scientific research. It can help farmers and horticulturists to understand the acidity and alkalinity of the soil, so as to guide the application of fertiliser, irrigation and crop management. In addition, soil pH sensors can be used to monitor soil contamination, assess soil health and study chemical processes in soil ecosystems.
Caution:
Although soil pH sensors can provide very useful real-time data, the following precautions need to be taken when using them:
1. soil pH sensors need to be calibrated periodically to ensure accurate measurements.
2. the sensor's electrodes may be susceptible to other ions in the soil, so measurements may not be accurate enough under certain soil conditions.
3. Proper techniques and methods need to be followed when collecting and analysing soil samples to ensure validity and reproducibility of results.
Through proper use and maintenance of soil pH sensors, they can effectively help manage and maintain soil health, which in turn improves crop yield and quality.
Related recommendations
Sensors & Weather Stations Catalog
Agriculture Sensors and Weather Stations Catalog-NiuBoL.pdf
Weather Stations Catalog-NiuBoL.pdf
Related products
 Combined air temperature and relative humidity sensor
Combined air temperature and relative humidity sensor Soil Moisture Temperature sensor for irrigation
Soil Moisture Temperature sensor for irrigation Soil pH sensor RS485 soil Testing instrument soil ph meter for agriculture
Soil pH sensor RS485 soil Testing instrument soil ph meter for agriculture Wind Speed sensor Output Modbus/RS485/Analog/0-5V/4-20mA
Wind Speed sensor Output Modbus/RS485/Analog/0-5V/4-20mA Tipping bucket rain gauge for weather monitoring auto rainfall sensor RS485/Outdoor/stainless steel
Tipping bucket rain gauge for weather monitoring auto rainfall sensor RS485/Outdoor/stainless steel Pyranometer Solar Radiation Sensor 4-20mA/RS485
Pyranometer Solar Radiation Sensor 4-20mA/RS485
Screenshot, WhatsApp to identify the QR code
WhatsApp number:+8615367865107
(Click on WhatsApp to copy and add friends)
